Background
Hip replacement surgery has been around since the early 1960s. Sir John Charnley experimented in the early 1950s, and he used a small (22 mm) stainless steel ball on a stem in 1962 that he inserted into the femur (hip) bone to replace the femoral head (ball). He then inserted a high-density plastic socket to replace the acetabular (socket) side of the hip joint. Both were secured with a self-curing acrylic polymer known as bone cement.
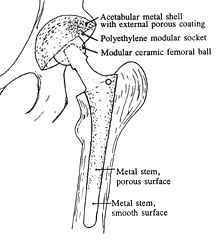
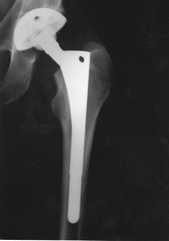
| Today, the modular balls are made of a cobalt-chrome metal alloy or a ceramic material, and some of the components are press-fit and do not require bone cement. The procedure remains basically the same: (1) the femur bone is amputated to remove the femoral head; (2) the femoral canal is reamed-out for insertion of the stem; (3) an acetabular socket is affixed to the socket side of the hip; and (4) the ball joint is inserted into the acetabular socket. This is known as a total hip replacement, or more correctly, total hip arthroplasty (THA). | image from www.wmt.com (Click on image to enlarge) |
| image from www.jri-oh.com (Click on image to enlarge) | image from www.jri-oh.com (Click on image to enlarge) |
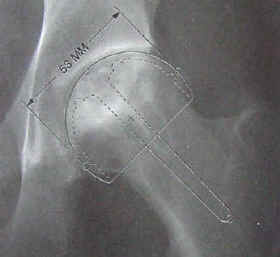
| The acetabular socket used in THA is normally lined with a high molecular weight polyethylene (sometimes the liner is ceramic). A metal or ceramic ball is attached to the stem and rotates within the socket. Fine particulate debris is produced from the wearing process of the ball against the liner that leads to tissue reaction. The body’s immune system attacks the debris, and consequently, attacks the adjacent bone supporting the THA device, leading to bone loss and a loosening of the device. This bone loss is known as osteolysis. To lessen the amount of wear, a small ball (approximately 30 mm) is used; however, the small size of the ball makes the joint less stable and increases the risk of dislocation in certain circumstances. The loosening of the THA device requires revision surgery in which a larger diameter stem must be inserted in the femoral canal. Depending on the age and activity of the patient, multiple revision surgeries may be necessary throughout a patient’s life. A young (under 60), active individual can expect only 10 – 15 years before needing revision surgery. Revision surgery can be complex and costly. The lifespan of a THA device is clocked in miles rather than years. (Note: Wright Medical Technology, Inc. has developed a large femoral head using metal-on-metal technology (see Hip Surface Replacement below) that reduces the risk of dislocations and osteolysis in THRs. The large head THR has received FDA approval and is actively being marketed.) |
| Hip Surface Replacements Although it was experimented with and attempted in the 1960s, metal-on-metal “resurfacing” of the femur and acetabulum was abandoned because of loosening of the fittings. With the refinement of acrylic fixation and its very successful use with the THA stem, interest in hip resurfacing was renewed, and it was subsequently used in several countries in the 1970s. (See History of Hip Resurfacing.) Resurfacing has the advantage of preserving the femoral bone stock (and marrow contained in the femur). It also has the advantage of easy future revision to THA if it becomes necessary. Since the femur is persevered and not amputated in the initial hip surface replacement surgery, it is available to support a THA stem should revision become necessary. Maintaining the integrity of the femur bone also aids in the mechanical transfer of weight and stress in a more natural manner. Where THA patients often experience thigh pain, recipients of hip surface replacements avoid that particular discomfort. |
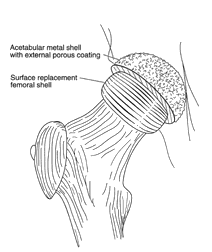
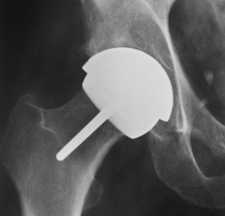
| image from www.jri-oh.com (Click on image to enlarge) | image from www.jri-oh.com (Click on image to enlarge) |
| Using a metal acetabular socket as well as a metal cap over the femur head (metal-on-metal) eliminates the polyethylene debris produced in THA. The metal wear debris from a hip surface replacement produces smaller particles than polyethylene wear debris. The inflammatory response to metal debris is considerably less than that from polyethylene debris. It is believed that the body can partially dissolve and expel metal since it is a naturally occurring substance in the body. There is concern by some of the toxicity of metal, but there is currently no definitive evidence that metal ions cause cancer. Since a metal surface does not wear as readily as a polyethylene lining, a larger ball (approximately 38-51 mm) can be used that adds stability to the joint and reduces the danger of dislocation. | images from www.wmt.com & www.jri-oh.com (Click on images to enlarge) |
| The surgery time for hip surface replacement is slightly longer than that for THA. The attachment of the acetabular socket is basically the same. It is press-fitted and does not require bone cement. The attachment of the cobalt-chrome cap requires a more precise alignment, and it takes slightly longer to fit. The hole for the pin insertion must be aligned and drilled, and the dome of the femoral head must be ground and shaped to fit the cap. Some bone cement is used to affix the cap, but the interior surface of both the cap and the socket is such that bone grows into the relief surface to grip the device. |
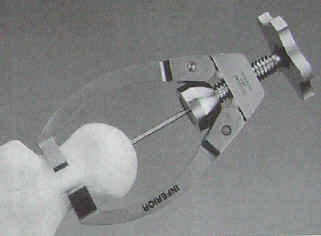
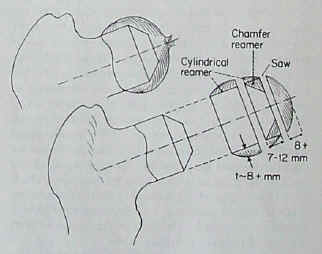
| The following images are from an Instructional Lecture delivered at an International Symposium in Fukuoka, Japan on March 16, 1996 by Harlan C. Amstutz, Peter Grigoris, and Frederick J. Dorey entitled "Evolution and future of surface replacement of the hip." Journal of Orthopaedic Science. J Orthop Sci (1998) 3:169-186. Superimposed hemisurface. Pin centering guide. Cylindrical reamer. Saw cutoff guide and oscillating saw. Chamfered reamer. Femoral head bone preparation. (Click on images to enlarge) |
| Risks involved in the hip surface replacement surgery are the same as the risks involved in any major surgery. Risks specific to the hip surface replacement involve the potential for cracking in the neck of the femur bone due to the drilling of the guide hole through the neck for the support pin in the metal cap, and also a negative reaction of the femur head to dislocation and being reshaped to fit the metal cap leading to the development of avascular necrosis (bone death)--often referred to as AVN--due to a disruption of blood circulation to the femur head and neck (see AVN Risk). In such instances, a THA could easily be performed to correct the problem. Hip surface replacement in the United States has been pioneered by Harlan C. Amstutz, M.D. at the Joint Replacement Institute in Los Angeles, CA. For years, a hip surface replacement in the United States has been labeled an “investigative device” by the Food and Drug Administration (FDA). The longest study has been conducted by Wright Medical Technology, Inc. under the product name of CONSERVE ® Plus Total Resurfacing Hip System. The clinical trials have proceeded for a number of years, and they are nearing their end. They have involved nine surgeons across the country in California, Florida, Texas, Maryland, North Carolina, Ohio, and in the Pacific Northwest. Corin Medical, Ltd. of the United Kingdom has also begun an FDA study in the United States using the Cormet 2000 device. Click here to read the 2-6 year follow up report of the first 400 CONSERVE ® Plus hips. In Europe, the Birmingham Hip Surface Replacement System (BHR) has been in use for many years. Smith & Nephew Inc., manufacturer of the BHR, applied for FDA approval, and perhaps due to the long record of use in Europe, they obtained FDA premarket approval to begin commercial distribution of their device in the United States on May 9, 2006 (see FDA approval letter and FDA announcement). Because the BHR was not previously used in the United States, the number of American surgeons qualified to use it was limited due to the fact that they had all been participating in the Wright and Corin studies; however, that is destined to change with the FDA approval obtained by Smith & Nephew. |







is there another solution for AVN other than operation ?
ReplyDeletehow can we treat a patient who is not willing for operation ?
wht r the risk if AVN has not been operated.